Neurofeedback for ADHD
Straight to:
Neurofeedback is a gentle, non-pharmacological, and painless therapy used to successfully treat the attention deficit hyperactivity disorder - ADHD. Professionally carried out by psychotherapists, child and adolescent psychiatrists or occupational therapists, Neurofeedback not only reduces the symptoms, but it also tackles the fundamental causes of the disease. People who are affected learn to recognize and control their brain activity permanently.
The following pages introduce Neurofeedback as a method of therapy. The earlier children and adults are treated, the better they and their families can learn to live with this challenge and to manage their everyday lives.
What is Neurofeedback?
Neurofeedback therapy is a very gentle, painless form of therapy used to treat ADHD. It is performed by occupational therapists, psychologists, or psychotherapists and not only fights against symptoms, but also combats the causes of the disorder. In contrast to medicine, this therapy can be used from as early as preschool children to adults.
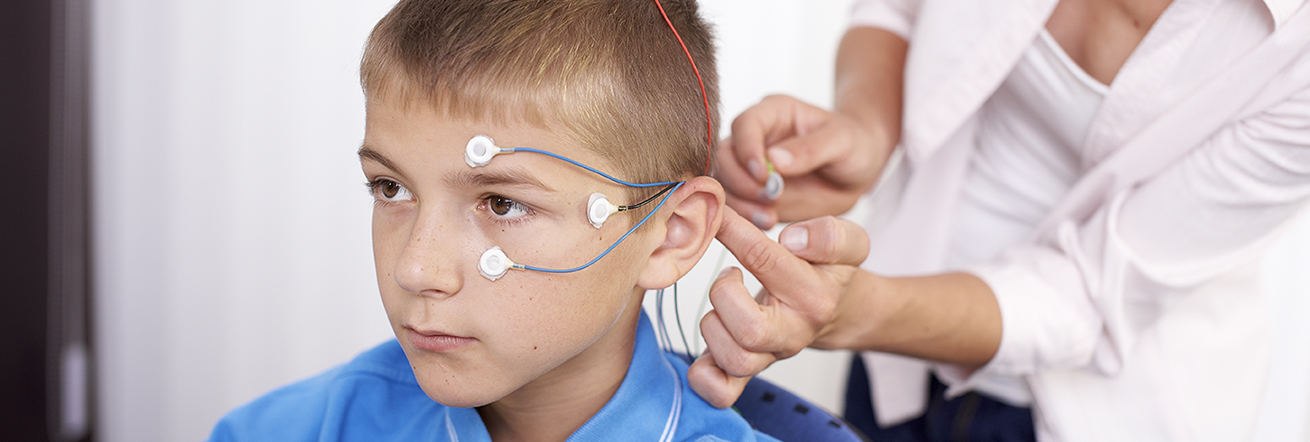
The scientific basis of this method is biofeedback: During these, changes in body processes such as the heart rate or blood pressure are made visible using devices. In the case of Neurofeedback, brain activity is measured and “reported back” to the patient via monitor.
Aim and Effect of Neurofeedback
The aim of Neurofeedback is to empower patients to influence their own brain activity so that they can process stimuli in a more controlled manner and perform tasks better. This is done by learning to voluntarily produce negative or positive shifts of the electric signals measured over a certain area of the brain, meaning that they should alternately put themselves into an attentive and a relaxed state.
The long-term effect of Neurofeedback is based on positive reinforcement: If they get their brain activity moving in the right direction, then this is immediately displayed on the monitor and the person receives a visual reward. Therefore, participants gradually learn to automate their behaviors and to transfer these to everyday life situations.
Neurofeedback Methods
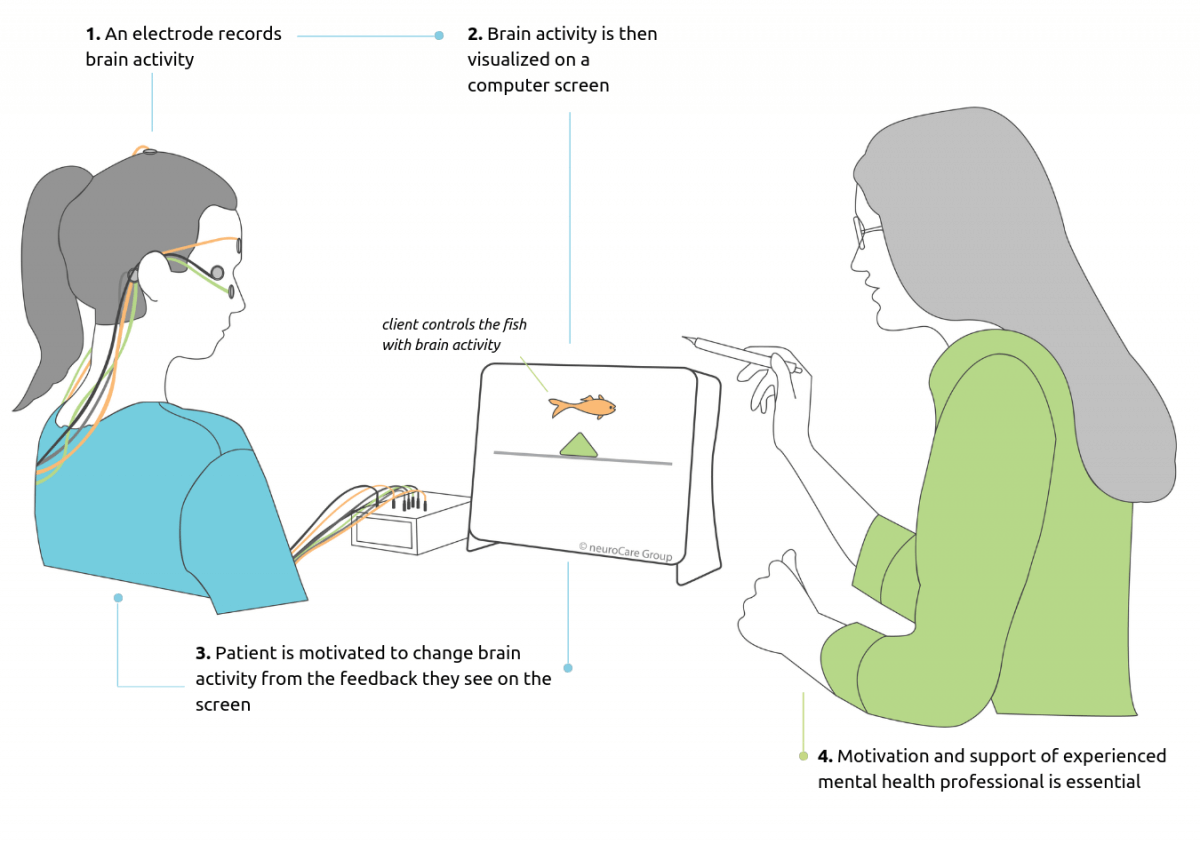
How does SCP Neurofeedback work for ADHD?
In people with ADHD, the regulation of SCPs works less well and especially the activation of brain activity, takes place at a lower level. This means that they have less energy available to process stimuli and cope with an upcoming task. This in turn then leads to inattention and impulsivity.
Find a clinic
Seek innovative and personalized treatment at a clinic near you
I’m a clinician
Learn more about our DTP, innovative technologies and training academy for health professionals.
I’m an investor
Learn more about our group and our mission to deliver innovative healthcare worldwide.
Stay up to date with our DTP & clinics
Stay up to date with our DTP & clinics
Copyright © neurocare group AG 2024